Two days ahead of the vaccination drive, the Ministry of Health & Family Welfare has sent a comprehensive fact sheet to the states for both the vaccines — Covishield and Covaxin — containing information regarding vaccine rollout, physical specification, dosage, cold chain storage requirements, contraindications and minor AEFIs (Adverse event following immunization).
Listing the contraindications, the health ministry cautioned against the administration of the vaccine in persons with a history of an anaphylactic or allergic reaction to a previous dose of COVID-19 vaccine, and in those with immediate or delayed onset anaphylaxis or allergic reaction to vaccines or injectable therapies, pharmaceutical products, food items, among others.
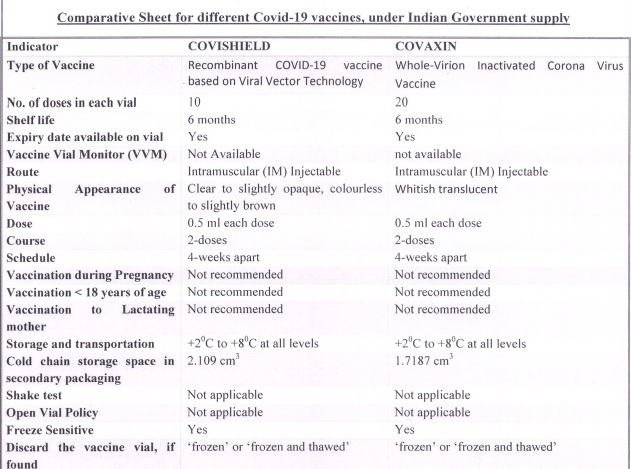
The letter, from Additional Secretary Dr. Manohar Agnani to the states, also included a comparative sheet for both the vaccines – Covishield and Covaxin – which contains information on vaccine platform, dosage, storage requirements, side effects, etc.
As per the directions, the vaccine will be administered only to those who are above 18 years of age. Women who are pregnant or not sure of their pregnancy and lactating mothers should not receive the vaccine, the Centre said in the letter.
The rulebook also said that interchangeability of the two vaccines is not allowed. “The second dose should also be of the same COVID-19 vaccine which was administered as the first dose,” it said.
The DGCA recently granted emergency use authorisation to two vaccines, Oxford’s Covishield being manufactured by Serum Institute of India and Bharat Biotech’s Covaxin. Both vaccines, the statement from the Health Ministry said, have established safety and immunogenicity.
The government also said that there will be a gap of 28 days between two doses of COVID-19 vaccine and its effectiveness will begin 14 days after the second dose.
Vaccination will have to be deferred for four to eight weeks after recovery of patients with active Covid symptoms, those who have been given plasma therapy, and those who are unwell and hospitalised for any other reason.
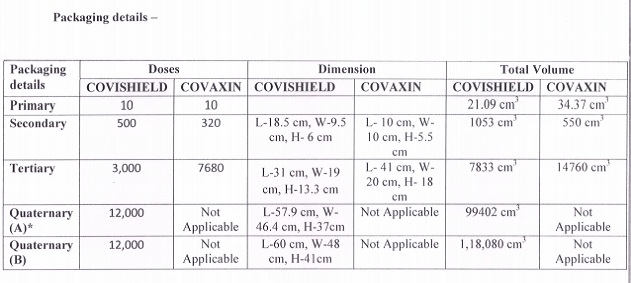
Here is the factsheet on the two vaccines shared by the Centre:
Prime Minister Narendra Modi will launch India’s COVID-19 vaccination drive on January 16 via video conferencing and adequate doses of the two made-in-India vaccines have been delivered across the country to all states and Union Territories, the government said on Thursday.
Prime Minister Modi will launch the pan-India rollout of the COVID-19 vaccination drive on January 16 at 10:30 AM via video conferencing, the PMO said.
A total of 3,006 session sites across all states and UTs will be virtually connected during the launch and around 100 beneficiaries will be vaccinated at each session site on the first day, the statement said.
The vaccination programme will use Co-WIN, an online digital platform developed by the Union Health Ministry, which will facilitate real-time information of vaccine stocks, storage temperature and individualised tracking of beneficiaries for COVID-19 vaccine.
This digital platform will assist programme managers across all levels while conducting vaccination sessions, the PMO said.
A dedicated 24×7 call centre – 1075 – has also been established for addressing the queries related to COVID-19 pandemic, vaccine rollout and the Co-WIN software.












